Biofilms are surface-bound communities of single-celled organisms that, collectively, have many characteristics more commonly associated with multicellular organisms. Biofilms are of great practical importance because they cause most persistent bacterial infections and can damage industry infrastructure.
Biofilms arise from bacteria colonizing the surface of a substrate which can range from living tissues to abiotic materials. Although mechanosensing properties of eukaryotes have been studied, the impact of mechanical properties in the environment on bacterial behavior remains obscure. Mechanosensing can be defined as translating mechanical cues from the environment into a biological response. Recently, there has been a highlight on the role of mechanosensitive proteins in cell envelopes of bacteria in sensing mechanical changes in their environment. A current project in the lab seeks to understand how Pseudomonas aeruginosa, Staphylococcus aureus, and other bacteria sense changes in their environmental stimuli by varying the stiffness of the substrate surface the biofilms are grown on. Understanding how substrate stiffness impacts early biofilm formation has applications in developing robust medical implants and material sciences.
We have recently shown that bacteria sense and respond to the mechanics of the surface to which they are attached. For this, we used a method we developed for quantitative, calibrated measurement of bacterial signaling response using confocal microscopy.
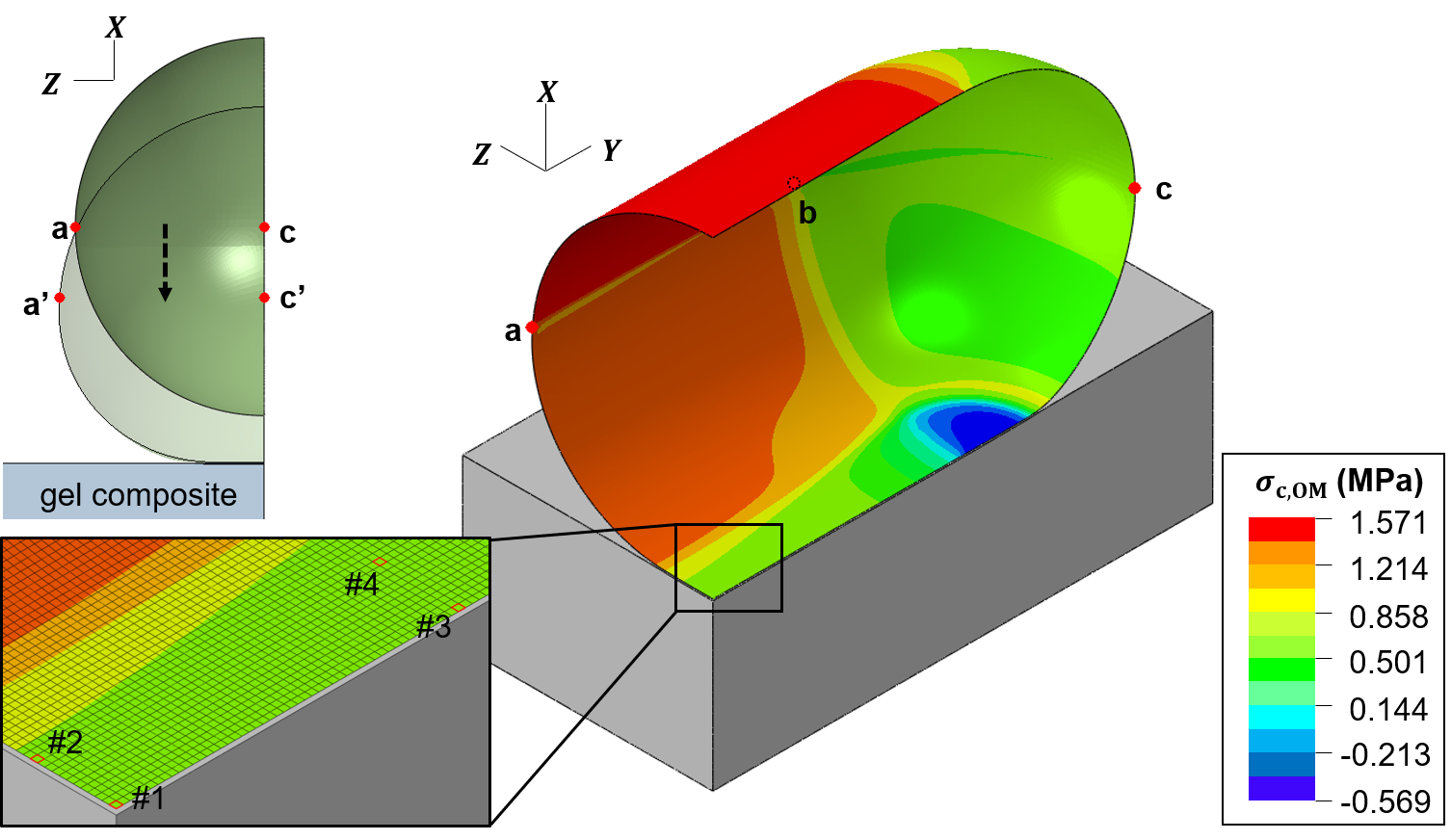
Chad Wong, Mechanical Engineering PhD student, did this finite-element modeling to describe the changes in mechanical stress and strain that a bacterial cell envelope experiences when it attaches to a surface, and how the changes in the bacterial cell envelope depend on the stiffness of the surface.
Earlier, we showed that bacteria “know” when they are attached to a surface by sensing mechanical shear stress.

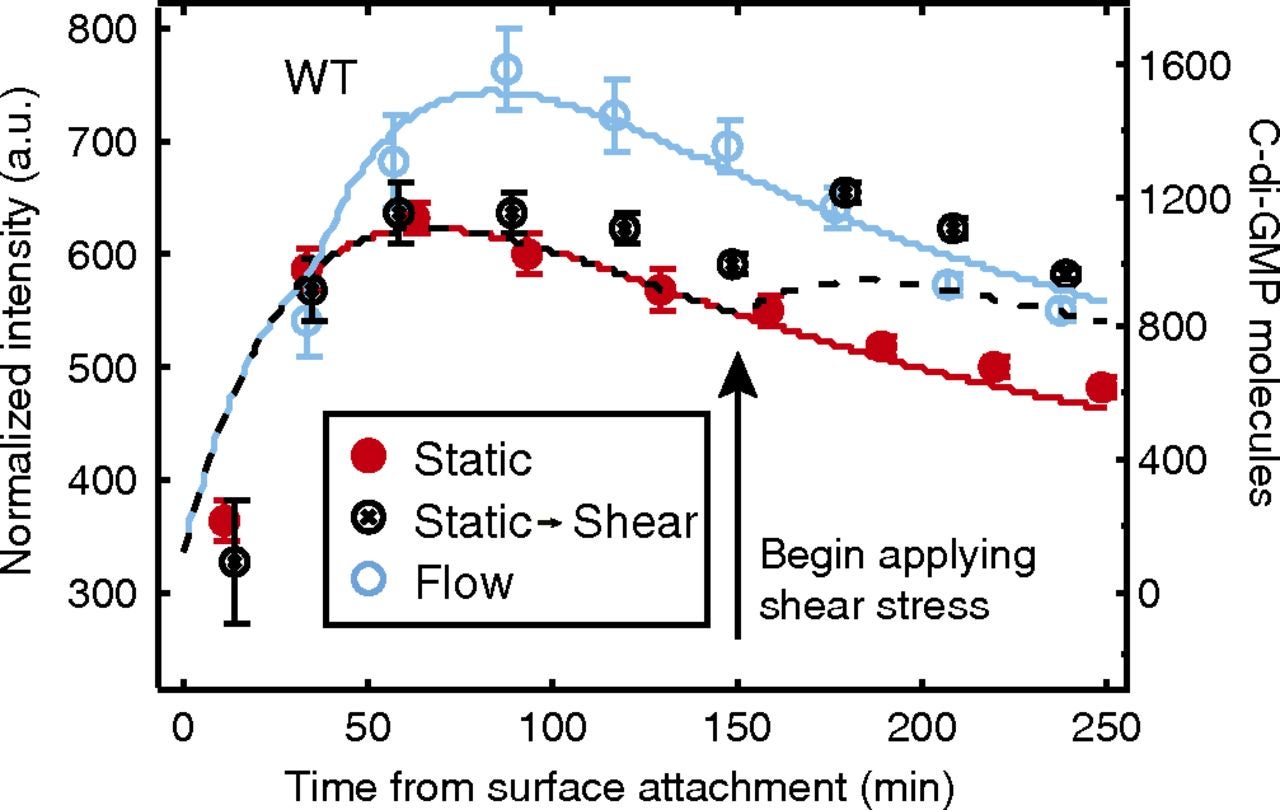
Chris Rodesney, Physics PhD student, did analytical modeling to describe his experimental measurements of bacterial signaling as a function of shear stress. Experimental data for three different stress timecourses are shown as discrete points of three different colors; modeling results for three different stress timecourses are shown as lines of the corresponding colors. https://www.pnas.org/doi/10.1073/pnas.1703255114
The whole field of bacterial mechanosensing is in its infancy, and there are a great many unaddressed questions to be explored. This contrasts strikingly with the well-developed field of eukaryotic mechanosensing. Our primary focus now is to understand more about how bacteria sense and respond to the mechanics of the substrate to which they are attached, and how we could develop surface materials that prevent bacteria from getting the mechanical cue(s) that they need to “know” that it’s time to develop into a biofilm. This is a new approach to biofilm prevention that would target a fundamental biological signaling mechanism and therefore, we hope, would be difficult for bacterial to evolve to circumvent.
People currently working on this project are Rida Siddiqi (undergraduate physics major) and Yu-Chern Chad Wong (Mechanical Engineering Ph.D. student). Postdoc Liyun Wang and undergraduate physics majors Mara Eccles and Macy Bell worked on this in the recent past. This work was started by Ph.D. student Chris Rodesney, who took a job at Intel after he graduated with his Ph.D.



